Two Patch Repair of Rastelli’s Type-A Complete Atrioventricular Septal Defect with Relief of Right Ventricular Outflow Tract Obstruction under Mild Hypothermic Extracorporeal Circulation and Cardioplegic Arrest: A Video Presentation.
Ujjwal K Chowdhury, Sukhjeet Singh, Niwin George, Lakshmi Kumari Sankhyan, Avinavsingh Chauhan, Sheil Avneesh, Andrei Jha, Vishwas Malik, Sreenita Chowdhury
Department of Cardiothoracic and Vascular Surgery, All India Institute of Medical Sciences, India
Corresponding author
Ujjwal K. Chowdhury, Department of Cardiothoracic and Vascular Surgery, All India Institute of Medical Sciences, India
Received: September 18, 2021
Accepted: September 30, 2021
Published: October 1, 2021
1. Abstract
1.1.Background: The association of right ventricular outflow tract obstruction with complete atrioventricular septal defect is uncommon and poses surgical challenge at the time of intracardiac repair. We report here-in a 18-months-old male child diagnosed with severe right ventricular outflow tract obstruction, complete atrioventricular septal defect and severe left atrioventricular valvular regurgitation undergoing successful resection of the right ventricular outflow tract with reconstruction of the complete atrioventricular septal defect by twopatch technique with limited transannular patch.
1.2.Keywords: Complete atrioventricular septal defect; Endocardial cushion defect; Right ventricular outflow tract obstruction; Two-patch technique.
Introduction
Atrioventricular septal defect is an appropriate designation for those hearts whose diverse morphology is unified by a deficiency of the atrioventricular membranous and muscular septa [1]. Abnormal differentiation and remodelling of the cushion mesenchyme into valvuloseptal tissue is the mechanism for development of atrioventricular septal defects [2]. The wide variability in the degree of development of the endocardial cushions explains the variability in size and extent of the septal defects and varying grades of malformed atrioventriculvar valves [3].
Several anatomical features are shared among all types of atrioventricular septal defects [1, 4, 5]. These include:
a) absence of the usual wedged position of the aortic valve due to a common atrioventricular valve ring;
b) lengthened outlet septum-to-ventricular apex ratio, resulting in an elongated left ventricular outflow tract and a “goose-neck” appearance;
c) shortened dimension of the inlet septum-to-ventricular apex giving the interventricular septum a “scooped out” appearance;
d) apical displacement of the attachments of the atrioventricular valves to the ventricular crest;
e) inferior displacement of the atrioventricular node and coronary sinus; and
f) variable degrees of underdevelopment of the inlet septum, resulting in absence of a ventricular septal defect, a restrictive ventricular septal defect, or a large ventricular septal defect [1, 4, 5].
There are a number of morphological factors that complicate the “usual” form of atrioventricular septal defect. These include: a) significant underdevelopment of either the right or left ventricle (prevalence of 10%)[6,7]; b) left ventricular outflow tract obstruction[8,9]; c) parachute deformity of the left atrioventricular valve[9]; d) double orifice left atrioventricular valve[10]; and e) double outlet left or right atrium [11].
The Rastelli classification describes 3 types of complete atrioventricular septal defects based on the morphology of the superior bridging leaflet, its degree of bridging, and its chordal attachments. The classification does not relate to anatomy of the inferior bridging leaflet. In a Rastelli type A defect (69%), the common superior bridging leaflet is effectively split into two “halves” at the septum. Rastelli type B (9%) is rare and describes an anomalous papillary muscle attachment from the right side of the ventricular septum to the left side of the common superior bridging leaflet. In type C defects (22%) there is marked bridging of the ventricular septum by the superior bridging leaflet. The superior bridging leaflet is not divided but floats freely over the ventricular septum without chordal attachment to the crest of the ventricular septum [12-14].
Patients with complete atrioventricular septal defect often present with severe congestive heart failure in the first 2 to 4 months of infancy and should be operated on between the ages of 3 and 6 months.15 Presently, early primary repair is the procedure of choice in the majority of the centres. The principles of surgical management of complete atrioventricular septal defect include closure of the atrial septal defect, closure of the ventricular septal defect, creation of two non-stenotic competent atrioventricular valves and avoidance of damage to the atrioventricular node and bundle of His [15-18].
Repair of the complete atrioventricular septal defect has been described using 3 different techniques: a) the single-patch technique, as described in 1962 by Maloney and Gerbode[17,18]; b) the two- patch technique as described by George Trusler in 1976[19]; c) the modified single-patch technique of Graham Nunn in 1995[20, 21].
One of the keys to successful repair of atrioventricular septal defect using any technique is intraoperative assessment using transesophageal echocardiography and after cardiotomy. This includes an assessment of the right and left ventricular size, the size and shape of the atrial and ventricular septal defect, the number and location of the papillary muscles, the arrangement and attachment of the chordopapillary apparatus [13, 15-21].
Classic single-patch technique
A pericardial, polytetrafluoroethylene or Dacron patch have been used in the single-patch technique. Use of pericardium as the ventricular septal defect patch is associated with the risk of development of aneurysm at the ventricular level [22]. The Dacron patch carries the risk of postoperative hemolysis [16-18].
Two-patch technique
A patch of Dacron or polytetrafluoroethylene is used to close the ventricular septal defect and a separate pericardial patch is used to close the atrial septal defect [19].
Modified single-patch technique
The ventricular septal defect is closed by placing a series of pledget supported 5-0 polypropylene sutures in the right-side of the interventricular septum. These sutures are then passed sequentially through the superior and inferior bridging leaflets at a site predetermined to separate the mitral and tricuspid valves. The sutures are then placed through the edge of the patch of autologous pericardium [20, 21].
Literature documents almost similar results of all 3 surgical techniques. The single-patch technique has been extensively employed at Children’s Hospital, Boston,15 the University of California, Los Angeles23 and Vanderbilt University.24 The operative mortality was 3%, 7% and 15% and the reoperative rates for mitral regurgitation was 9%, 6% and 6% respectively among 3 institutions [15,23,24].
In a meta-analysis of results from five institutions among 794 patients undergoing operation by two-patch technique, the operative mortality was 7% and the incidence of reoperation for mitral regurgitation was 8% [16]. Among 72 consecutive repairs using modified single- patch technique, the operatively mortality was 2.8%, mild left atrioventricular valve regurgitation 29% and moderate regurgitation in 5% [20,21]. A trivial ventricular septal defect that did not require reoperation was noted in 20% of patients [21].
We report here-in the surgical repair of the complete atrioventricular septal defect using two-patch technique and resection of right ventricular outflow tract obstruction. An 18-months-old male child presented with feeding difficulty with repeated episodes of chest infection, poor weight gain and cyanosis since birth. Clinically, he had cardiomegaly, left parasternal heave and ejection systolic murmur of grade IV/VI intensity over the 2nd and 3rd intercostal spaces. The non-invasive oxygen saturation at rest was 84-85% as determined by pulse oxymetry. Cross sectional echocardiography established the diagnosis of complete atrioventricular septal defect, severe left atrioventricular valvular regurgitation and severe diffuse right ventricular outflow tract obstruction with peak systolic gradient of 100 mmHg. The obstruction was at the level of pulmonary valve and right ventricular outflow tract.
The child underwent successful resection of the right ventricular outflow tract with reconstruction of the complete atrioventricular septal defect by two-patch technique and limited transannular unfixed pericardial patch.
Although our institution is a tertiary level center, the socioeconomic profile of the patients and lack of health insurance benefit led to delayed referral and surgery.
Surgical Techniques
Following median sternotomy, the thymus was subtotally excised taking care not to expose the brachiocephalic vein. The pericardium was opened on the left side raising a right-sided flap in between stay sutures using scissors and not cautery to avoid inadvertent cautery- induced ventricular fibrillation.
The operation was performed under moderately hypothermic cardiopulmonary bypass with an aortic infusion cannula, angled venous cannula into superior vena cava and a straight venous cannula into the inferior vena cava. St. Thomas based cold hyperkalemic blood cardioplegia (1:4) and topical ice cooling was used for myocardial preservation.
The persistent ductus arteriosus was clipped using a Hemaclip (Johnson and Johnson Ltd., Ethicon, LLC, San Lorenzo, USA) after pulling down the superior surface of the pulmonary artery at the commencement of cardiopulmonary bypass as described by Dwight McGoon. The pump flow was temporarily lowered at the time of interruption of the ductus arteriosus.
The main pulmonary artery was opened in between stay sutures prior to cross clamping the aorta. Exposure was achieved through an oblique incision in right atrium 1 cm posterior to the interatrial groove. Four stay sutures were placed on the right atrial edges. The left ventricle was vented through the atrial septal defect using a DLP vent (Medtronic Inc., Minneapolis, MN). The anatomic features of the malformation were examined. The coronary sinus and margin of the atrioventricular septal defect delineate the boundaries of the atrial level defect. There was a prominent cleft between the left and right superior bridging leaflets, consistent with Rastelli type A classification and a large ventricular septal defect with scooped out ventricular septum.
The stenosed pulmonary valve was incised at the level of pulmonary valvular commissures. Obstruction of the right ventricular outflow tract was relieved by excising the parietal insertion of the infundibular septum. Relief of right ventricular outflow tract obstruction was achieved by combined trans-rightatrial, transpulmonary approach. Adequacy of right ventricular outflow tract resection was checked by visual assessment and insertion of an appropriate sized Hegars dilator.
The atrioventricular valve was distended by injecting cold saline into the ventricular cavities. This enabled us to delineate the optimal coaptation point of the left superior and inferior bridging leaflets and identify any abnormalities in valve coaptation that require attention during reconstruction.
Two elastomer vascular loops were used to retract the left superior and inferior bridging leaflets. The length and height of the ventricular septal defect was measured. The height of the septal patch was determined between the crest of the ventricular septum and the optimal point of coaptation of the left superior and inferior bridging leaflets. The shape of the ventricular septal patch is classically “D-shaped” with its convexity along the edge of the ventricular septum. It is important to maintain a smaller height of the ventricular septal patch to prevent iatrogenic postoperative left atrioventricular valvular regurgitation.
Multiple pledget supported interrupted 5-0 polypropylene sutures (Johnson and Johnson Ltd., Ethicon, LLC, San Lorenzo, USA) were placed 0.5 cm away from the ventricular septal crest.
Extreme precautions were taken at superior and inferior corners of the ventricular septal defect, where the septum meets the annulus to prevent residual ventricular septal defect. At these points, the sutures were brought through the annulus and the substance of the superior and inferior bridging leaflets to prevent residual ventricular septal defects in these locations.
The D-shaped Dacron polyester patch (Bard® Savage® filamentous knitted polyester fabric, Bard Peripheral Vascular Inc., Tempe, AZ, USA) slightly smaller than the size of the ventricular septal defect was used to close the defect.
For reconstruction of the leaking septal commissure, a stay suture of 6-0 polypropylene was placed at the free leaflet margin opposing the atrial edge of the coaptation border. Multiple interrupted, non- pledgeted 6-0 polypropylene sutures were used to repair the septal commissure taking precautions to take the bites through the atrial edge and not the ventricular edge, as recommended by Alain Carpentier, thus ensuring perfect competence [13].
Cold saline was injected again into the left ventricular cavity to check for a competent left atrioventricular valve and to identify any additional leaking commissures. Following repair of the left atrioventricular valve, we ensured that the left atrioventricular valve opening commensurate with the indexed mitral valve orifice, thus avoiding iatrogenic mitral stenosis.
The left atrioventricular valve was then “sandwiched” between the top of the ventricular septal patch and a second patch of pericardium to be used to close the atrial level defect.
Throughout the operation, interrupted mattress sutures were used. Precautions were taken at both superior and inferior portion of the defect to make certain that the ventricular septal patch was brought into the corner of the superior and inferior bridging leaflets and then along its border so as not have any residual defects.
The pericardial patch was sutured to the edge of the ostium primum defect with the exception of the area of bundle of His, where the sutures were displaced towards the superior edge of the coronary sinus, thus diverting the coronary sinus to the left side. Before completing the suture line, the left side of the heart was filled with iced saline and gentle ventilation to evacuate residual air, if any.
Cold saline was injected into the right ventricle to ensure competence of the right atrioventricular valve. The right atrium was closed in two layers using 5-0 polypropylene suture. The patient was weaned off cardiopulmonary bypass with stable hemodynamics.
The postoperative peak systolic right ventricle-to-left ventricle pressure ratio was 0.5. Intraoperative transesophageal echocardiography did not demonstrate any residual ventricular septal defect. There was no left or right atrioventricular valvular regurgitation.
Short- and Long-term Results
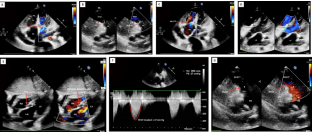
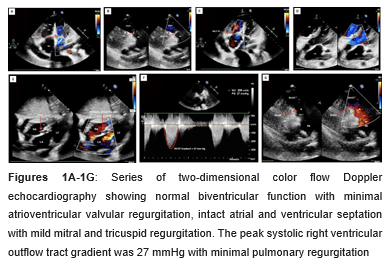
Postoperatively, the child was in normal sinus rhythm and recovery was uneventful. At 16th month follow-up the child was asymptomatic, no clinical evidence of cardiac failure, with Ross’s clinical score of 2. Two-dimensional color flow Doppler echocardiography revealed normal biventricular function with minimal atrioventricular valvular regurgitation, intact atrial and ventricular septation with mild mitral and tricuspid regurgitation. The peak systolic right ventricular outflow tract gradient was 27 mmHg with minimal pulmonary regurgitation (Figures 1A-1G).
Conclusions
Surgical repair of severe right ventricular outflow tract obstruction with complete atrioventricular septal defect should be done in early childhood, before the onset of severe deformation of the atrioventricular valves. In cases of severe left atrioventricular valve regurgitation, the repair should be undertaken earlier to prevent further deterioration of valve function, left ventricular dilatation and function. Adequate resection of the right ventricular outflow tract without creating pulmonary regurgitation is of paramount importance in providing good long-term outcome.
References
1. Abbruzzese PA, Livermore J, Sunderland CO, et al. Mitral repair in complete atrioventricular canal. Ease of correction in early infancy. J Thorac Cardiovasc Surg. 1983; 85:388-395.
2. Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995; 77:1-6.
3. Van Praagh R, Litovsky S. Pathology and embryology of common atrioventricular canal. Prog Pediatr Cardiol. 1999; 10:115-127.
4. 4Anderson RH, Zuberbuhler JR, Penkoske PA, Neches WH. Of clefts, commissures and things. J Thorac Cardiovasc Surg. 1985; 90:605-610.
5. Jacobs JP, Burke RP, Quintessenza JA, et al. Congenital Heart Surgery Nomenclature and Database Project: Atrioventricular canal defect. Ann Thorac Surg. 2000; 69 (suppl):36.
6. Freedom RM, Bini M, Rowe RD. Endocardial cushion defect and significant hypoplasia of the left ventricle: A distinct clinical and pathological entity. Eur J Cardiol. 1978; 7:263-281.
7. Corno A, Marino B, Catena G, Marcelletti C. Atrioventricular septal defects with severe left ventricular hypoplasia. J Thorac Cardiovasc Surg. 1988; 96:249-272.
8. Chang CI, Becker AE. Surgical anatomy of left ventricular outflow tract obstruction in complete atrioventricular septal defect. A concept for operative repair. J Thorac Cardiovasc Surg. 1987; 94:897-903.
9. De Biase L, Di Ciommo V, Ballerini L, Bevilacqua M, Marcelletti C, Marino B. Prevalence of left-sided obstructive lesions in patients with atrioventricular canal without Down syndrome. J Thorac Cardiovasc Surg. 1986; 91:567-472.
10. Ilbawi MN, Idriss FS, DeLeon SY et al. Unusual mitral valve abnormalities complicating surgical repair of endocardial cushion defect. J Thorac Cardiovasc Surg. 1983; 85:697-704.
11. Suzuki Y, Hamada Y, Miura M, Haneda K, Horiuchi T, Ogata H. Double-outlet left atrium with intact ventricular septum. Ann Thorac Surg. 1988; 45:332-334.
12. Rastelli G, Kirklin JW, Titus JL. Anatomic observations on complete form of persistent common atrioventricular canal with special reference to atrioventricular valves. Mayo Clinic Proc. 1966; 41:296.
13. Carpentier A. Surgical anatomy and management of the mitral component of the atrioventricular canal defects. In Anderson RH, Shinebourne EA, editors. Pediatric Cardiology. 1979; 466- 490.
14. Torfs CP, Christianson RE. Anomalies in Down syndrome individuals in a large population-based registry. Am J Med Genet. 1998; 77:431-438.
15. Hanley FL, Fenton KN, Jonas RA, et al. Surgical repair of complete atrioventricular canal defects in infancy. Twenty-year trends. J Thorae Cardiovasc Surg. 1993; 106:387.
16. Backer CL, Mavroudis C, Alboliras IT, et al. Repair of complete atrioventricular canal defects: results with the two-patch technique. Ann Thorac Surg. 1995; 60:530.
17. Maloney IV Jr, Marable SA, Mulder DC. The surgical treatment of common alrioventricular canal. J Thorac Cardiovasc Surg. 1962; 43:84.
18. Gerbode F. Surgical repair of endocardial cushion defect. Ann Chir Thorac Cardiovasc. 1962; 1:753.
19. Trusler GA: Discussion of Mills NL, Ochsner JL, King TD. Correction of type C complete atrioventricular canal. Surgical considerations. J Thorac Cardiovasc Surg. 1976; 71:20.
20. Nicholson IA, Nunn GR, Sholler GE, et al. Simplified single- patch technique for the repair of atrioventricular septal defect. J Thorac Cardiovasc Surg. 1999; 118: 642.
21. Nunn G. AV canal repair: without VSD patch. Congenital Heart Disease Symposium, Eighty-first Annual Meeting. The American Association for Thoracic Surgery. 2001.
22. Burkhart, HM, Moody SA, Ensing GH, et al. Ventricular septal aneurysm after atrioventricular septal repair with pericardium. Ann Thorac Surg. 1996; 61:1838.
23. Capouya ER, Laks H, Drinkwater DC Jr, et al. Management of the left atrioventricular valve in the repair of complete atrioventricular septal defects. J Thorac Cardiovase Surg. 1992; 104:196.
24. Merrill WH, Hammon JW Jr, Graham TP Jr, et al. Complete repair of atrioventricular septal defect. Ann Thorac Surg. 1991; 52:29.