Upper pole first laparoscopic left donor nephrectomy: single surgeon experience from four centers in Jordan and Bahrain.
1. Abstract
1.1.Background: The first laparoscopic donor nephrectomy was reported by Ratner in 1995. Subsequently, the procedure has been adopted by most transplant centers by replicating the open technique using either a total laparoscopic or hand-assisted technique.In 2011, Tunc reported the first direct upper pole kidney access in laparoscopic radical nephrectomy, with a reverse technique that started the dissection from the upper pole towards the renal hilum. We elected to apply a modification of this new technique to laparoscopic donor surgery to assess efficacy and safety.Methods: Retrospective analysis of sequential cohorts of 36 cases performed in 4 hospitals by the same surgeon over a period of 12 months compared to the previous 28 cases done by the same surgeon using a conventional laparoscopic technique.
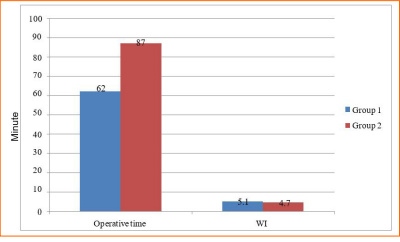
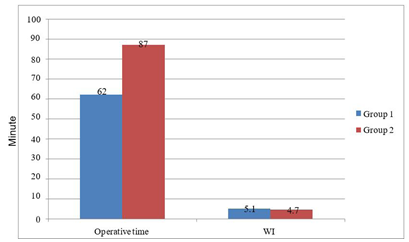
1.2.Results: Mean operating time was lower with the upper pole first technique (62 ± 11 minutes vs. 87 ± 13 min) whereas warm ischemia times were similar between two groups (5 min vs 4.7 min). Mean recipient post-operative serum creatinine levels (0.92 vs 1.04 mg/dl) were likewise similar.. Mean blood loss was minimal in both groups2.Results: Mean operating time was lower with the upper pole first technique (62 ± 11 minutes vs. 87 ± 13 min) whereas warm ischemia times were similar between two groups (5 min vs 4.7 min). Mean recipient post-operative serum creatinine levels (0.92 vs 1.04 mg/dl) were likewise similar.Mean blood loss was minimal in both groups(<50 cc) with no transfusion requirement, and no conversion to open surgery in either group.
1.3.Conclusion: upper pole first laparoscopic donor nephrectomy is safe and a slightly faster method compared to the conventional total laparoscopic technique with similar outcomes.
(1) Eye Specialty General Hospital, Amman, Jordan
(2) Salmaniya Medical Complex, Manama, Bahrain
(3) Al-Khaldi Medical Center
(4) Istiklal Hospital
(5) Albashir Medical Center
(6) Specialty Hospital, Amman, Jordan
Introduction
The first reported laparoscopic nephrectomy was performed by Clayman in 1991 [1]. However, the first laparoscopic donor nephrectomy was not performed until 1995 at the John Hopkins Bay View Medical Center by Ratner [2]. The procedure was accomplished by using four ports with extraction of the kidney through a lower midline abdominal incision. Warm ischemia time (WIT) was less than 5 minutes and the donor was discharged on the first post-operative day with no complications. The recipient experienced immediate graft function [2]. Since this milestone achievement, laparoscopic donor nephrectomy was eventually adopted by most centers and has become the standard of care because of the advantages in the donor of early post-operative recovery, less post-operative pain, better cosmetic results, equivalent graft functional outcomes, and better motivation for living donors of meniscal injury, and degenerative joint disease (6, 7). ACL reconstruction is performed to stabilize the knee joint and resume normal function to the full extent. ACL reconstruction is carried out using such graft options as autograft, allograft, and artificial ligaments (8). Autograft refers to a tissue transplanted from one location within the body of a person and grafted into another site on the same individual, such as the bone-patellar tendon-bone (BPTB) and the hamstring (HT) (9). Hamstring-tendon grafts are one of the most popular choices for anterior cruciate ligament reconstructions. Semitendinosus and gracilis tendons were initially used together as two single strands. Hamstring tendon graft application has recently increased due to its relatively low donor site morbidity (10).
To accept “minimally invasive” surgery[3,4,5]. The original surgical technique described by Ratner was modified by transplant surgeons (including placement of the extraction incision) in order to achieve better cosmetic results and reduce WIT. For example, some surgeons started using an Endocatch bag to help reduce incision size and protect the kidney during extraction, while others used direct extraction aiming to reduce WIT. Other modifications included making the extraction incision either in the para- umbilical or Pfannenstiel location instead of using a lower midline incision [6, 7].
The surgical technique for laparoscopic nephrectomy using the basic steps reported previously by Clayman [1] were dissection starting with colon mobilization, then identification of the ureter with dissection of the lower pole of the kidney, followed by dissection of renal artery and vein, then division of vascular branches and upper pole dissection(1). In 2005, Porpiglia described their experience with direct access to renal artery at the Treitz ligament during laparoscopic radical nephrectomy (that follows the classical steps for radical nephrectomy) with similar results in terms of blood loss, operating time, and outcomes [8, 9, 10].
In 2011, Lutfi Tunc published a description of his modified upper pole direct access technique with early ligation of the vascular pedicle for laparoscopic radical nephrectomy. He performed en bloc ligation of the renal pedicle in 86% of cases, with significant reductions in operating time and good surgical outcomes [11].
In this study, we adopted the Direct upper pole technique for left laparoscopic transperitoneal living donor nephrectomy, leaving the vascular dissection as the last step of the procedure instead of early direct pedicle occlusion to study the effect of this technique in living donor kidney transplantation.
Materials and Methods
Between June 2018 and June 2019, we performed 36 transperitoneal direct upper pole access left laparoscopic donor nephrectomies, another two cases using same technique for right laparoscopic donor nephrectomy were excluded due to small number. All were performed by a single surgeon (Al-Geizawi) at four medical centers including three centers in Jordan (Albashir Medical Center, Istiklal Hospital and Al- Khaldi Medical Center) and one center in Bahrain (Salmaniya Medical Complex). We retrospectively compared the outcomes in these 36 cases to 28 cases that were performed using the traditional laparoscopic technique in the period (June 2017 to May 2018) immediately prior to the study period. All donors were evaluated by transplantation team according to protocol of investigations including computerized tomographic angiography.
Trocar Placement:
In all cases, patients were placed in the right lateral position on a straight table, with 20 degrees tilt to the left side, insufflation was done using a closed technique in which a Verez needle was placed at the tip of the left 10th rib. Following insufflation, we used the location of the upper trocar (2 cm from the tip of 9th rib) as a landmark, a 10 mm periumbilical trocar was placed, and then under direct vision 10 mm left lower and 5 mm left upper trocars were placed. In a few selected cases, a fourth 5 mm trocar was placed under the xiphoid for retraction.
Traditional Surgical Technique:
In the first 28 “control” cases, the surgical steps were: 1- mobilization of descending colon and spleen; 2-identification of left ureter; 3- Dissection of renal artery and vein; 4- division of gonadal, lumbar and adrenal branches; 5- Upper pole dissection; and 6- direct extraction of the kidney through a Pfannensteil incision.
Upper Pole First Technique:
In the next 36 cases, we applied the upper pole first technique, in which we used the same lateral position with similar trocar placement. We once again started with dissection of the colon, but then switched to upper pole dissection until we identified the lumborum muscle and released all of spleno- renal attachments. Next, the left adrenal gland was dissected from the upper pole of the kidney and the adrenal vein was ligated with release and exposure of the superior border of the left renal vein. We then shifted to the lower pole of the left kidney where the left ureter was identified and dissected along with the gonadal vein. The gonadal vein was followed up to the inferior border of left renal vein and then divided. By pulling the ureter up, easy rotational movement of the left kidney was accomplished so the left renal artery could be visualized and dissected from its origin on the aorta. With the dissection completed, we created a Pfannenstiel incision while keeping the peritoneum intact. The Click’aV Plus TM, polymer clip Grena-UK was applied to the left renal artery, followed by a proximal titanium clip (Endo Clip TM 10 mm Medtronic). The left renal vein was then secured with two Click’a Plus TM polymer clips Grena UK. The kidney was extracted manually with aid of the laparoscopic vision through the Pfannenstiel incision. Time of surgery was calculated from time of insufflation to the time of kidney extraction.
All procedures were performed by a single surgeon, port closure was done under laparoscopic vision, and extraction site was closed by assistant surgeon or resident. A surgical drain was placed in one case in the conventional laparoscopic technique group and two cases in the (upper pole first) technique group according to surgeon assessment. Group 1 refers to the conventional laparoscopic technique group and Group 2 refers to the (upper pole first) technique group.
Results
Mean donor age was 28.6 ± 4 years in Group 1 and 29.2 ± 6 years in Group 2 (P=0.28). The youngest (age 19) and oldest (age 52) donors were both in Group 1.8 donors in group 1 were males (28.5%) while 14 males in group 2 (38.9%). 2 donors in Group 1 had two arteries and one donor in Group 2 had two arteries. Mean length of hospital stay for donors was 4.2 days in Group 1 and 4.4 days in Group 2. Estimated blood loss was less than 50 cc in both groups with no need for transfusions in any donor and no conversion to open surgery in either group.
We found a significant difference in mean operating time in favor of the (upper pole first) technique, (Group (1) 87 ± 13 minutes vs. Group (2) 62 ± 11 minutes, p=0.0143) (graph 1, table 1). Mean WIT was not statistically different (5.1 min vs. 4.7 min. for Groups 1 and 2, respectively P=0.68). Mean serum creatinine levels for recipients with functioning grafts at one week and 6 months follow-up in Group 1 were 1.04 ± 0.2 and 1.35 ± 0.35 mg/dl, respectively, and 0.94 ± 0.25 and 1.27 ± 0.42 in Group 2, respectively (P=0.39). There were no major donor surgical complications in either group. There was no donor mortality whereas one recipient in Group 1 died on post-operative day 20 with severe viral pneumonia. There was one graft loss in Group 2 secondary to accelerated acute rejection with subsequent graft removal on post- operative day 3 after failure of medical treatment.
Discussion
Laparoscopic living donor nephrectomy has become the preferred method for renal procurement in most centers as it provides similar outcomes in terms of graft function with superior results in terms of post-operative pain and earlier return to normal activity that provides an incentive to promote living donation [12, 13, 14].
Since the procedure was described by Ratner in 1995 [2], which used similar steps to the procedure for simple laparoscopic nephrectomy described by Clayman [1] in 1991, the order of surgical steps was not modified for living donor nephrectomy except for the way in which the kidney was extracted [6].Even with single port trans-umbilical donor nephrectomy, the dissection steps follow the same sequence [15, 16].
Applying a modification of the Tunc technique [11] (direct upper pole access) described for laparoscopic radical nephrectomy to living donor laparoscopic nephrectomy appears to reduce operating time, which will have a favorable effect on operative costs[17] and possibly protects the kidney from potential deleterious effects of prolonged pneumoperitoneum with reductions in renal blood flow[18,19] .
The Tunc technique was described for laparoscopic radical nephrectomy or nephro- ureterectomy, and was shown to significantly reduce operating time. When we applied the modified technique for laparoscopic left donor nephrectomy, we also found a significant reduction in operating time even though this initial experience represents our “learning curve” with this technique. The other observation we have made is that direct upper pole dissection takes the surgeon directly to the left adrenal gland and left adrenal vein, which is ligated earlier during the course of surgery. Ligation of the adrenal vein makes visualization and identification of the renal vein and gonadal vein much easier. Once the gonadal vein is identified, we directly dissect at the level of the lower pole of the kidney, which facilitates identification of the ureter, a step that sometimes takes a long time when we try to dissect the ureter distally in obese patients in conventional laparoscopic technique. Direct upper pole dissection makes it easier to have more rotational movement of the kidney when the ureter and lower pole are lifted up, as the upper pole is freely mobile, which subsequently makes it easier to ligate lumbar veins when present, It also makes the dissection of the renal artery at its origin from the aorta easier with better exposure
Applying a metallic clip proximal to a Click’aV Plus TM protects against transmission of aortic pulsation to the polymer clip, which adds to security in addition to sparing a significant length of the artery that is usually lost when using a stapler.
A steep learning curve will always be an issue, especially when performing laparoscopic nephrectomy., In general, a minimum of fifteen cases are required for trainees to perform laparoscopic radical nephrectomy[20]. However, in the case of laparoscopic donor nephrectomy, the number of cases will be higher as more technical skills are required to achieve appropriate vascular dissection with minimum manipulation and traction on the renal artery and vein.
In our study, we did not include right laparoscopic donor nephrectomy because during the study period we only performed 2 right donor nephrectomies. We applied same technique for right kidneys and it seemed easier, but as the number is so small we excluded right laparoscopic nephrectomies from our analysis.
There seem no functional outcome difference between both groups which is expected as warm ischemia time was similar in both groups, reducing the operative time in upper pole first group did not carry short term functional outcome difference.
Conclusions
Left upper pole renal pole first technique during laparoscopic donor transperitoneal nephrectomy appears to be a safe approach that facilitates surgery with equivalent short- term outcomes. This technique needs to be studied further with a larger number of cases by multiple surgeons in order to validate consistency of results.
References
1. Clayman RV, Kavoussi LR, Soper NJ, et al. Laparoscopic nephrectomy: initial case report. J Urol 1991;146:278
2. Lloyd E. Ratner, Lars J Ciseck, Robert J Moore, et al. Laparoscopic live donor nephrectomy, Urol 1995: 1047 – 1049
3. Simforoosh N, Basiri A, Tabibi A, et al. Comparison of laparoscopic and open donor nephrectomy: a randomized controlled trial. BJU Int. 2005;95(6):851-855.
4. Leventhal JR, Kocak B, Kokkinos C, et al. laparoscopic versus open live donor nephrectomy in renal transplantation: a meta-analysis . Ann Surg. 2008;247(1):58- 70
5. Nicholson ML, Kaushik M, Lewis GR, et al.Randomized clinical trial of laparoscopic versus open donor nephrectomy. Br J Surg. 2010;97(1):21-28.
6. Lloyd E Ratner, Michael Fabrizio, Kenith chavin , et al. Technical considerations in the delevary of the kidney during laparoscopic live-donor nephrectomy. Am Coll Surg. 1999;189:4:427-430.
7. Mathew C Rawlins, Thomas Hefty. Scott L Brown, et al. Learning laparoscopic donor nephrectomy safely, a report on 100 cases. Arch Surg. 2002:137: 531-535.
8. Porpiglia F, Terrone C, Cracco C, et al. Early ligature of renal artery during radical laparoscopic transperitoneal nephrectomy: description of standard techniqueand direct access. J Endourol. 2005;19: 623-627.
9. Porpiglia F, Renard J, Billia M, et al. Left laparoscopic radical nephrectomy with direct access to the renal artery: technical advantages. Eur Urol. 2006;49:1004-1010.
10. Porpiglia F, Terrone C, Cracco C, et al. Direct access to the renal artery at the level of Treitz ligamentduring left radical laparoscopic transperitoneal nephrectomy. Eur Urol. 2005;48:291-295.
11. Lutfi Tunc, Abdullah Erdem, Fazli Polat, et al. Direct upper kidney pole access and early ligation of renal pedicle significantly facilitate transperitoneal laparoscopic nephrectomy procedures: Tunc technique. Surg Laparosc Endosc Percut Tech. 2011;23:1965-1969.
12. Antcliffe D, Nanidis TG, Darzi AW, et al. A meta-analysis of mini-open versus standard open and laparoscopicliving donor nephrectomy. Transpl Int. 2009;22(4):463-474.
13. Oyen O, Anderson M, Mathisen L, et al. Laparoscopic versus open living-donor nephrectomy: experiences from a prospective randomized single-center study focusing on donor safety. Transplantation. 2005;79(9):1236-1240
14. Leventhal JR, Antcliffe D, Kokkinos C, et al. Laparoscopic versus open live donor nephrectomy in renal transplantation: a meta analysis. Ann Surg. 2008;247(1):58- 70.
15. Inderbir S Gill, David Canes, Monish Aron, et al. Single port transumbilical (E-NOTES) donor nephrectomy. J Urol .2008;180:637-641.
16. Rolf N Barth, Michael W Phelan, Lauren Goldschen, et al. Single port donor nephrectomy provides improved patient satisfaction and equivalent outcomes. Annals Surg 2013;257(3)527-533.
17. Kenneth T. Pace, Sarah J Dyer, Eric C paulin, et al. Laparoscopic v open nephrectomy: a cost- utility analysis of the initial experience at a tertiary-care center. J Endovasc . 2001;16(7): 495-508.
18. Scbastian Demy Henaere, Liane Feldman, and Gerald Fried. Effect of Pneumoperitoneum on renal perfusion and function: a systematic review. Surgical Endoscopy. 2007;21(2): 152-160.
19. London ET, Ho HS, Neuhaus AM, et al. Effect of intravascular volume expansion on renal function during prolonged CO2 pneumoperitoneum. Ann Surg. 2000;231: 195-201.
20. Jeon SH, Han KS, Yoo KH, et al. How many cases are necessary to develop competence for laparoscopic radical nephrectomy? . J Endourol. 2009;23: 1965-1969.